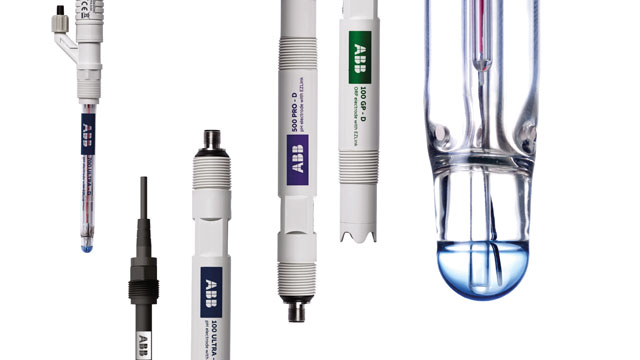
How to Calibrate a pH Sensor for Water for Reliable Results
Accurate measurement of water acidity or alkalinity is essential in a variety of applications, including environmental monitoring, industrial processes, and laboratory research. A pH sensor provides critical data for maintaining water quality, optimizing chemical processes, and ensuring compliance with regulations. However, even the most advanced sensors can produce inaccurate readings if they are not calibrated properly. Regular calibration ensures the sensor delivers reliable results and maintains long-term performance.
When preparing a pH Sensor for Water for use, calibration is a fundamental step. This process involves adjusting the sensor to match known reference solutions, which ensures that the readings accurately reflect the true pH of water samples. Without proper calibration, even minor deviations can lead to incorrect decisions in water treatment, industrial operations, or laboratory experiments.
Understanding pH Sensor Calibration
Calibration aligns the sensor’s readings with standard pH values, typically using buffer solutions of known pH. Most sensors require a two-point or three-point calibration to ensure accuracy across the expected measurement range. A two-point calibration uses two buffer solutions—commonly pH 4 and pH 7 or pH 7 and pH 10—while a three-point calibration adds an intermediate buffer for greater precision.
Regular calibration compensates for sensor drift, which can occur due to electrode aging, contamination, or environmental factors. Drift can lead to inaccurate readings if left unchecked, so following a consistent calibration schedule is crucial for maintaining sensor reliability.
Preparing for Calibration
Before calibrating a pH sensor, proper preparation is essential. Start by cleaning the sensor electrode to remove any residues or deposits that may interfere with accurate readings. Use distilled water to rinse the electrode and gently blot it dry with a lint-free tissue. Avoid rubbing the electrode, as this may cause damage.
Next, gather fresh buffer solutions that cover the pH range relevant to your measurements. Using expired or contaminated buffers can introduce errors, so ensure that the solutions are stored according to manufacturer recommendations. Also, check that the sensor’s temperature compensation is functioning properly, as temperature variations can influence pH readings.
Step-by-Step Calibration Process
Calibrating a pH sensor generally involves the following steps:
- Immerse the Sensor in the First Buffer: Place the electrode in the first standard solution, usually pH 7, and allow the reading to stabilize.
- Adjust the Sensor Reading: Use the calibration controls on the meter to set the reading to match the buffer’s known pH.
- Rinse and Repeat: Rinse the sensor with distilled water, then immerse it in the second buffer, such as pH 4 or pH 10. Allow the reading to stabilize and adjust as necessary.
- Optional Three-Point Calibration: For higher precision, repeat the process with a third buffer solution.
- Finalize Calibration: Rinse the sensor, return it to the measurement environment, and verify accuracy with a test solution if desired.
Following this procedure ensures that the sensor provides reliable readings across the full pH range.
Maintaining Accuracy Over Time
Even after proper calibration, regular maintenance is necessary to sustain sensor performance. This includes routine cleaning, avoiding exposure to harsh chemicals, and storing the electrode in an appropriate solution when not in use. Sensors that are left dry or exposed to extreme conditions may require more frequent recalibration.
It is also important to track calibration history and establish a schedule based on usage frequency, environmental conditions, and manufacturer recommendations. For critical applications, daily or weekly calibration may be necessary, while occasional monitoring may only require monthly checks.
Benefits of Properly Calibrated pH Sensors
A well-calibrated pH sensor offers several advantages:
- Accuracy: Ensures that measurements reflect the true pH of water samples.
- Consistency: Reduces variability between readings, especially when multiple sensors are used.
- Compliance: Supports adherence to regulatory standards in environmental monitoring and industrial processes.
- Process Optimization: Enables precise chemical dosing and process adjustments based on reliable data.
- Longevity: Extends the life of the sensor by reducing the impact of drift and contamination.
Proper calibration also instills confidence in data reporting, which is crucial for research, audits, and decision-making in water management.
Common Challenges in Calibration
Users may encounter common calibration challenges such as unstable readings, electrode contamination, or air bubbles on the sensor surface. Addressing these issues requires careful handling, thorough cleaning, and proper buffer preparation. Some advanced sensors come with self-diagnosis or automatic calibration features to simplify the process and improve accuracy.
Conclusion
Calibrating a pH sensor is a vital practice for achieving accurate and reliable water quality measurements. By following a structured calibration process, maintaining proper sensor care, and regularly verifying performance, operators can ensure consistent results across various applications. Properly calibrated sensors not only improve measurement accuracy but also support regulatory compliance, optimize processes, and enhance long-term sensor performance.